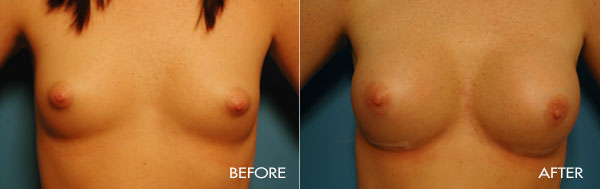
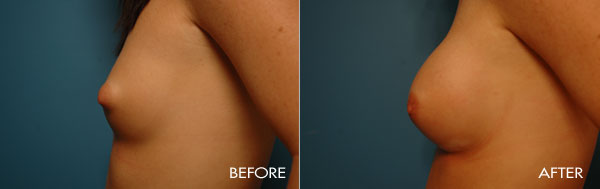
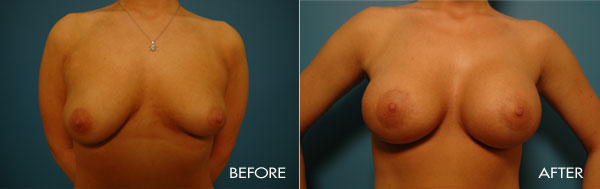
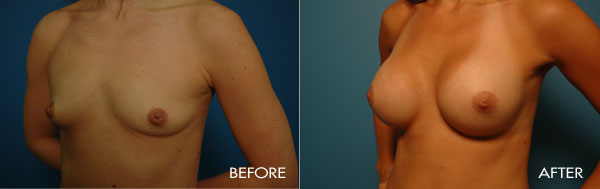
Breast augmentation surgery is an area of special clinical interest to Dr. Samuels and is one of our most commonly requested surgical procedures. Dr. Samuels trained at the prestigious University of Texas Southwestern (Parkland and Baylor Hospitals), the number-one plastic surgical residency training program for the last 20 years. In Dallas as well as in the first 10 years of her practice, Dr. Samuels not only trained with the surgeons who developed the new cohesive round and shaped silicone devices, but she also was one of only a handful of surgeons who participated in the clinical trials which culminated in the 2006 FDA approval of the new silicone gel devices used today.
Having been chosen among only a handful of U.S. providers to conduct clinical research on modern breast implants spanning two decades, Dr. Samuels does not believe that there is a single breast implant to suit all patients. Dr. Samuels uses round as well as shaped implants, smooth as well as textured saline and silicone gel breast implants, and implants from all three brand-name companies, rather than a single device or manufacturer. She uses a detailed, bio-dimensional approach based on each patient’s unique breast measurements and an assessment of soft tissue in order to select the best implant. As women, Dr. Samuels and her staff are uniquely equipped to understand the needs of the female patient seeking breast enhancement surgery.

Saline implants are filled with sterile saline, or salt water, which is a natural body substance. These implants are placed within the implant pocket and inflated with saline to the desired volume. While the incidence of rupture is low, it is slightly higher than with silicone gel due to the fluid nature of the filler as well as the presence of the fill port. Since salt water is absorbed by the body, a rupture may be more easily detected by the patient. Should a rupture occur, all brand-name implant manufacturers provide a lifetime warranty for replacement of the device. Because the implant is filled with fluid, the risks of rippling, wrinkling and heaviness in the lower pole of the breast are greater than with a silicone device. The more soft-tissue coverage provided, the less noticeable these findings will be. For this reason, most saline devices are placed beneath the pectoralis major muscle and are not as popular as their silicone gel counterparts.

Round and shaped, smooth and textured, silicone implants are filled with a gel that gained FDA approval in 2006. Unlike older liquid gel devices, the gel in these implants is thicker; rupture and contracture are infrequent. If a device fails, which is less likely than with a saline device, the gel is unlikely to leak into surrounding tissue. If a mammogram is suspicious for implant rupture, an ultrasound or MRI might be indicated. Cohesive round devices are available in three different projections; shaped and textured devices also exist. There are several different levels of cohesivity that affect how firm or how soft implants feel. Many women believe silicone gel devices feel softer and more like normal breast tissue than saline implants, with less chance of detectable rippling, wrinkling or edge palpability.

All three brand-name implant manufacturers have historically made anatomically shaped, more cohesive silicone gel implants, most of which gained FDA approval in 2006. Dr. Samuels is one of fewer than 30 surgeons in the U.S. selected to complete the clinical trial of some of these textured and shaped devices. The devices remain safe and are extensively studied and widely used in Europe and the U.S. Textured and shaped devices remain some of the best options for breast implant revision and reconstruction. Since Dr. Samuels was one of the few U.S. surgeons approved to use the early shaped and round high-profile devices, patients come from all over the U.S. for evaluation.
Shaped devices provide a great option for patients who want a more natural breast shape and for revision patients. Also, the more cohesive the filler, the more form stable its shape will be, and the lower the capsular contracture and rupture rates will be. In 2019, Allergan’s textured saline and silicone gel shaped devices were removed from the market due to a very low-risk association with a capsular disease process known as BIA-ALCL (breast implant associated-anaplastic large cell lymphoma) ALCL developed in the capsule of 0.03% of all textured devices. Sientra and Mentor’s risk of this rare disease is at least 6% lower than that of Allergan, so Sientra and Mentor still make a differently textured device that is available for those patients who would receive a better cosmetic result with a textured, shaped device than with a smooth round device. The nuances of which breast implant is best for each patient are carefully discussed at the time of your personal consultation. For more information on ALCL, please visit plasticsurgery.org or contact our office.

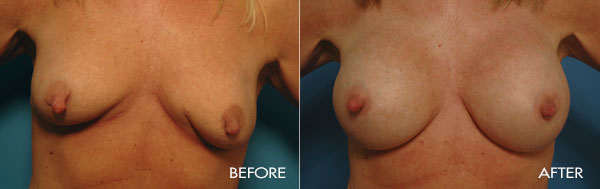
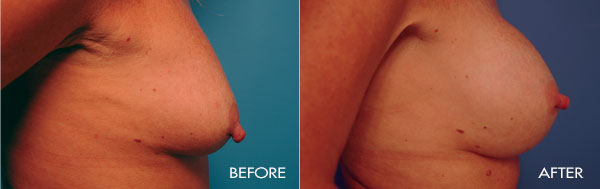
This surgery is usually performed on women whose breasts are undersized in proportion to the rest of their body, or women who have lost fullness due to pregnancy, aging or weight loss. The surgery may also be performed for congenital deformities or breast asymmetry.
For the most proportionally enhanced results, Dr. Samuels uses the bio-dimensional approach of matching implant to patient. Several different levels of cohesivity affect how firm or soft the various implants feel and how much upper pole fullness can be achieved. This requires measurement and matching of the patient’s own base diameter and sizing. This technique drastically reduces revision rates in breast augmentation.
The surgical procedure involves the insertion of an individually selected implant chosen to provide the patient’s desired fullness and shape and to mimic the look and feel of natural breast tissue. In addition to using the bio-dimensional approach to matching an implant to a particular patient, Dr. Samuels and her nursing staff will also review breast implant “wish pics” with all patients to ensure that the surgical goals are both achieved and realistic. The incision location is individual to each patient, but is typically concealed in the breast fold, axilla or areola.
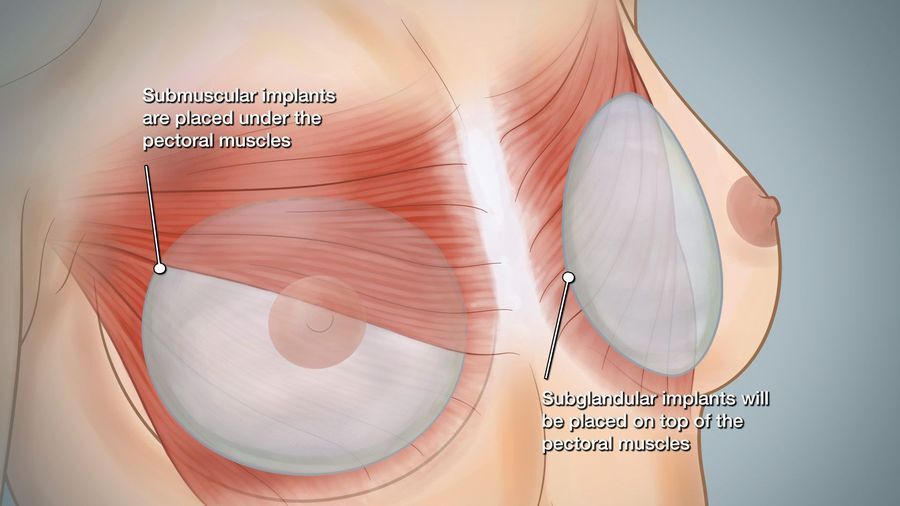
Implants may be placed on top of or beneath the pectoralis major muscle depending on individual characteristics of each implant and patient. Routine screening of implanted patients by mammography is done according to the recommendations of The American Cancer Society and may be supplemented by ultrasound or MRI for gel patients. You should tell your mammographic facility that you have breast implants so that additional views will be taken to adequately visualize the breast tissue. If this is done, breast cancer detection rates are unchanged after implant surgery. Likewise, breastfeeding is also possible following implant placement, but tell us if you plan to do this when we discuss surgical timing and incision selection.
Dr. Samuels and her staff will give you specific instructions regarding surgical preparation, including medications to avoid and nicotine cessation two weeks prior to surgery. The surgery is performed under local anesthesia with intravenous sedation, or with a light general anesthetic as an outpatient. Dr. Samuels has a fully Quad A accredited in-suite operating room.
While all surgeons do not utilize Quick Recovery Breast Augmentation, Dr. Samuels learned this carefully controlled technique while training in Dallas with the world’s leading breast surgeons. This technique not only reduces recovery to merely days, it also drastically reduces revision rates to less than 5%. View our blog postings on the Quick Recovery Technique and Revisional Surgery to learn more about them. All sutures are dissolvable, and patients may resume normal daily activities immediately.
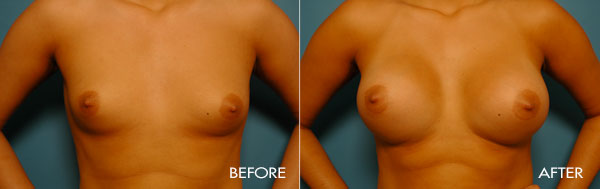
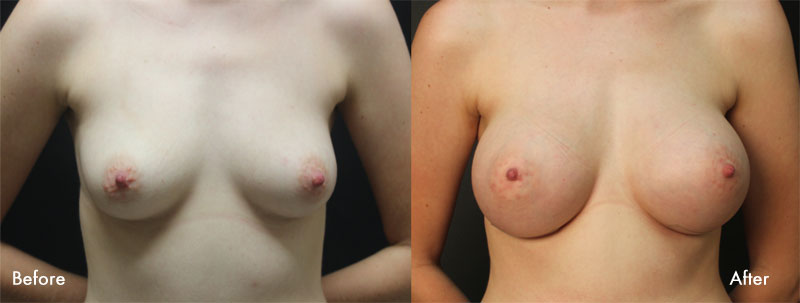
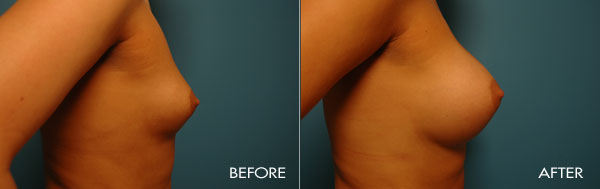
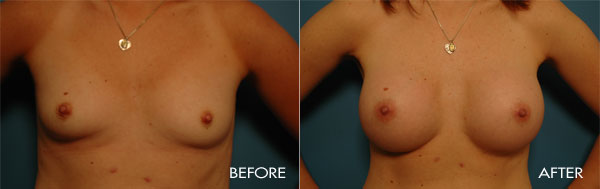
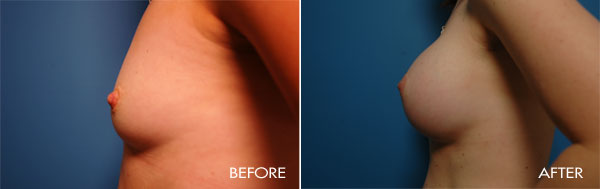
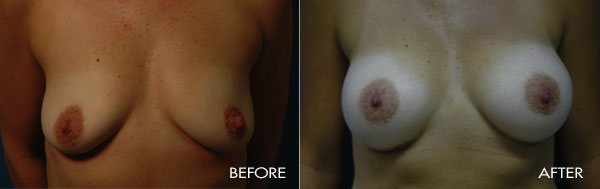
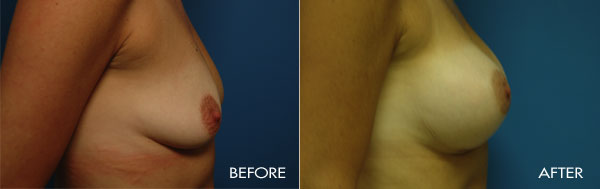

For more information about breast augmentation or to see if you’re a good candidate for this procedure, schedule a consultation with Dr. Samuels at 502-897-9411.
Our practice serves Louisville, KY and the surrounding areas.